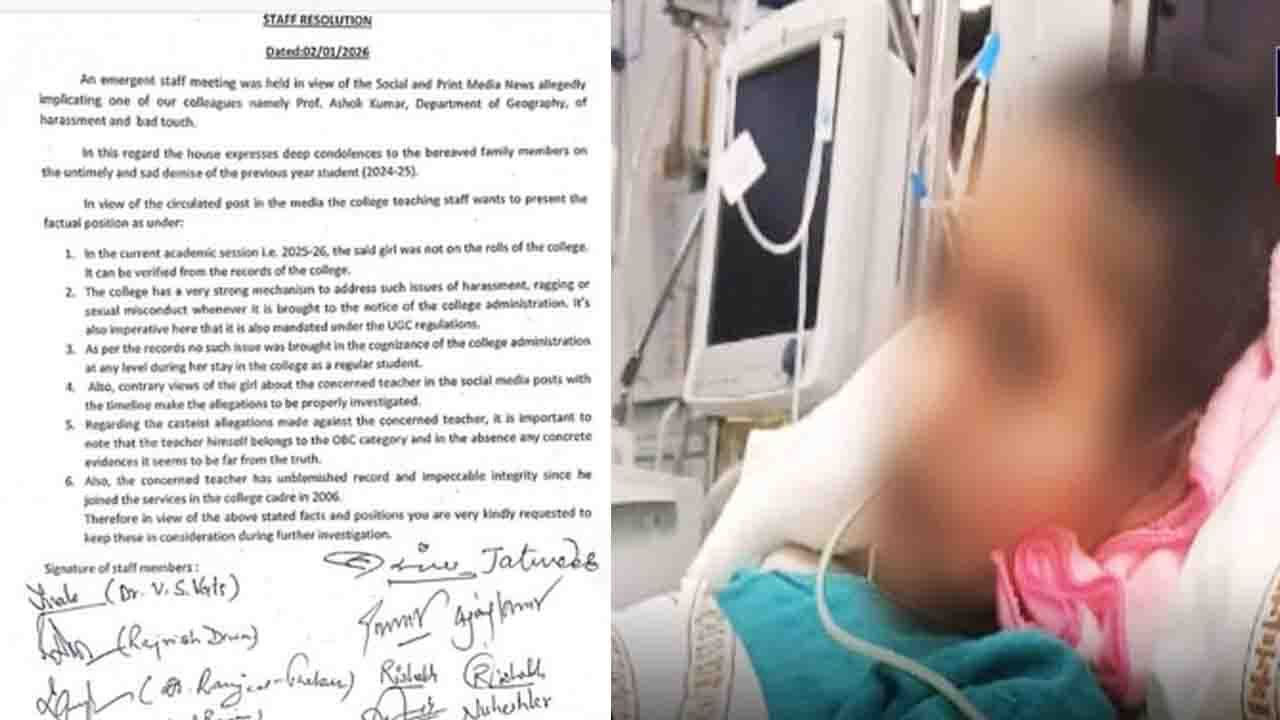
Himachal Drug Crisis: The Central Drugs Standard Control Organization (CDSCO) issued a drug alert for September 2025, reporting that 112 drug samples from across the country failed the quality test. Of these, 49 drugs manufactured in Himachal Pradesh failed the quality test. These included cough syrups, medicines for serious ailments like heart disease, cancer, diabetes, high blood pressure, asthma, and pain. One cough syrup was even found to be counterfeit.
According to the latest CDSCO report, 113 drug samples across the country failed, including 49 from Himachal Pradesh. Sixteen drugs from Gujarat, 12 from Uttarakhand, 11 from Punjab, 6 from Madhya Pradesh, and some from other states are also on this list. Several drugs from pharmaceutical industries in Himachal’s Sirmaur and Baddi regions have failed the test. Specifically, a cough syrup manufactured in Sirmaur, “Besto-Cough Dry Cough Formula,” is suspected to be counterfeit and is under investigation.
Eight drugs from Effi Parenterals Industries, located in Baddi, Himachal Pradesh, which is WHO, GMP, and ISO certified, failed the test. These included three samples of Telmisartan, a drug for high blood pressure, Ondansetron, used in chemotherapy, and four samples of Albendazole, a dewormer.
Similarly, samples of Clopidogrel and Aspirin (75 mg), a heart attack prevention drug, from G Laboratories, Sirmaur, failed the test. Telmisartan, a drug for high blood pressure and heart disease, from Bannet Pharmaceuticals, Baddi, and Bupivacaine, an anesthesia injection used in surgery, from Protech Telelink, Sirmaur, also failed the test. Cefpodoxime Projectil, a drug for bacterial infections for children and adults, from Upkar Pharmaceuticals, Sirmaur, also failed the test.
According to the CDSCO’s Drug Alert Report, the primary reason for 80 to 90 percent of drug samples failing is that the drugs are not manufactured in accordance with the Indian Pharmacopoeia (IP) and pH standards, resulting in their failure. Reasons for drug failure include low-quality raw materials and failure to store drugs at the prescribed temperature.
Himachal Drug Controller Dr. Manish Kapoor stated that most drugs were found to have minor defects, which are being rectified immediately. However, some drugs have major defects, for which show-cause notices will be issued to the industries. Additionally, all drugs from the failed batches will be recalled from the market. Furthermore, monitoring of pharmaceutical industries will be further increased.